In this article we share with you questions that were discussed on Jun 16, 2021 on our free webinar “Gender Selection, PGD, PGS in Surrogacy and IVF Programs”.
How safe is the testing for the embryo? Are there any precautions or circumstances in which it is not advised?
Naturally, because it is an invasive treatment, there may be stress and a risk to the embryo. However, because modern technologies have progressed so far, the risk is relatively low.
Different testing procedures exist, and they can be carried out in various stages.
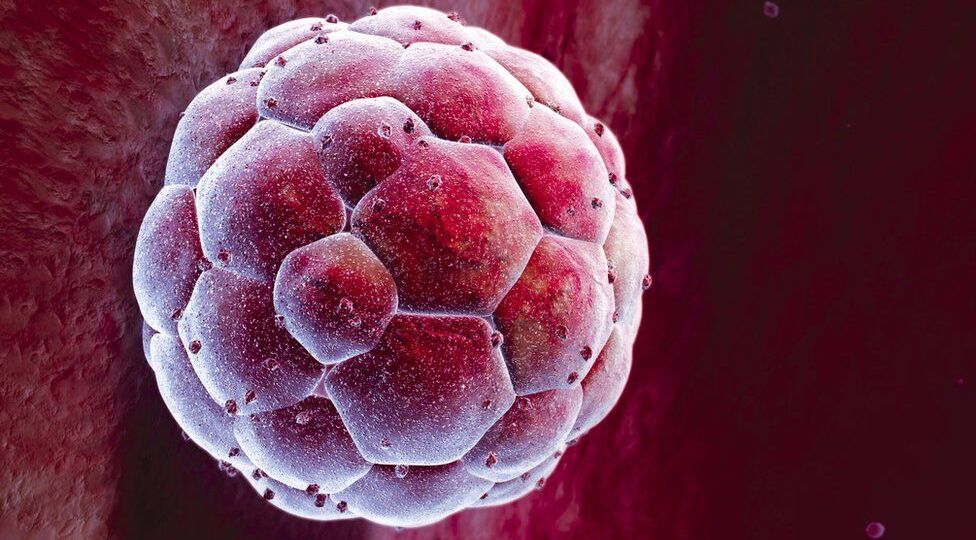
At ADONIS, we typically prefer to do PG testing on the 6th day when Trichoderma biopsy is being done and 1 to 6 cells are taken from the embryo for that.
It is also possible to do PG testing on the 3rd day, but in that case, you can only take 1 cell. So the validity of the test is being minimized significantly.
In this instance, there is no certainty that the embryo will develop into a full blastocyst.
It is not that the testing is harmful, but it is a necessary element of the embryo’s development.
It’s perfectly common for the number of embryos to differ from the number of blastocysts. Not every blastocyst matures into an embryo.
What’s the difference between testing methodologies? What is the difference between NGS and FISH testing?
Of course, when we’re talking about ART programs, the creative process is artificial. And we’ll never be able to fully comprehend what occurs in nature. The industry has already come as near as feasible, yet there are still differences between an embryo generated organically within a human body and one created artificially.
During the ovulation phase of a woman’s normal cycle, approximately 10 oocytes are produced naturally. And, through natural selection, the woman’s own body would decide which egg is the most powerful based on hormone levels and other factors.
And when we are using ART, during the egg retrieval there is an extra supply of hormones is going on. This means that all of those 10 eggs, have the potential of developing and all of them become viable even those that naturally are weaker, that naturally would be discarded and not survived. And for those eggs, it is not possible to identify visually if they are healthy genetically and do not have any deviations.
As those eggs are developing, the really weaker ones, they naturally might not develop to full blastocysts.
And of course, there is a sperm factor as well, and with the ICSI method, the best sperm morphologically is being selected. But it is not possible to do a genetic test on each sperm cell before.
So why many doctors recommend PGT, because there are some variables, that are not possible to detect in embryos even while they developing in perfect visual conditions. So that is why it is important to check hidden anapolies.
PGD is checking for full exposure of chromosomes.
The FISH methodology is faster and cheaper than NGS.
But we need to understand what is the purpose of testing. If we want to exclude those possible anomalies that developing during the first days of ART, then we recommend NGS 24 chromosomes testing. Because It allows you to do the check of full-spectrum chromosomes.
But if you know that in your condition you have a very specific chromosome anomaly that you do not want to pass to the embryo, then it is possible to use the FISH method. The methodology of FISH is more like a zond that can target a particular chromosome in this case.
So the reason the FISH method is very fast is that you can target a particular chromosome.
This method can be also used in a combination with karyotyping.
Can NGS and FISH methods be used at the same time?
No, NGS and FISH can not be used together. The reason for that is because they are going into different laboratories. One is molecular, the other is cellular. You can not do 2 biopsies in one embryo.
What should we be aware of when it comes to the vitrification process?
It is a relatively new process but advanced in the industry. The start of the process is to pull out the water of the embryo, so there will not be a risk of ice concentration that could erupt the embryo. Then it is being replaced with cryoprotection, which is replacing the water and creating a shell around oocytes or embryos.
It does certain stress on an embryo of course. So these protection levels are being introduced slowly in different concentrations so the embryo slowly is getting used to it. And once the embryo is protected by those cry protectors the embryo is being put into liquid nitrogen at such low temperature that all of the live processes have been paused. After that, it’s being put into a cryo carrier.
The survival percentage of embryos with the vitrification process is very high – over 99%. It is much more effective than old cryopreservation methods and it is very safe for eggs or embryos.
In comparison with sperm, vitrification is not used as a method, because there is no need to vitrify every cell. Because there is a huge quantity of sperm cells. So simple cryopreservation is being used for freezing sperm.
What is the thawing process? What kind of service providers are there? And what kind of media do we have in our laboratory?
The role of the media of those elements on thawing is to extract those cry protectors that have been added in a process of vitrification. All of them contain some type of polysaccharide.
So for the thawing process, we always like to use the same media that has been used for the vitrification.
At our laboratory, we have the media that are most commonly used: Cryotec, Kitazato, Irvine, and SAGE.
The type of preferred media can depend on a country.
But basically, there are only two types of polysaccharides that are used to take out the water off the embryo in all media. The role of that sugar is just to clean and bring the water. So there are more and more studies are being done to unify the process.
How can we be certain that biomaterial is safe and reliable?
Double control is one of the most used procedures. There are at least two people in charge of the process, not just one. Labeling, computerized control, and other features are also available.
Checking all of the documentation is also crucial from a patience standpoint.
Please, check the full recording of our webinar here:
